General Area 研究领域
Liquid Organic Hydrogen Carrier, Supercritical Fluid, Micro-nano Materials, Biomass Energy
有机液态储氢、超临界流体、微纳米材料、生物质能

2nd Generation Scale-up Continuous Apparatuses for LOHCs' Hydrogen Storage
Copyright@HydroTransformer Co. Ltd., http://www.hydrotransformer.com
第二代桌面式有机液态储氢连续式储放氢装置
版权@氢易能源科技有限公司,http://www.hydrotransformer.com

2nd Generation Skid-Mounted Pilot Prototype Machines (20‘ST)
for LOHCs' Hydrogen Storage
Copyright@HydroTransformer Co. Ltd., http://www.hydrotransformer.com
第二代20尺撬装式有机液态储氢载体中试样机
版权@氢易能源科技有限公司,http://www.hydrotransformer.com
1. Liquid Organic Hydrogen Carriers, 有机液态储氢技术(合作方:氢易能源科技,国电投广东分公司,红杉中国,上海重塑集团,国中基金)
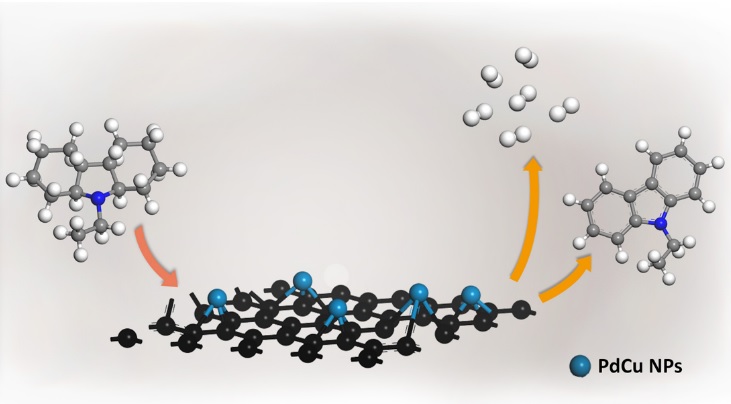
氢能作为清洁的新型能源,其制备、储运和应用各环节都是科学界和工业界的研究热点。有机液体储氢材料因为其突出的性能特点在集中式和分布式氢能应用的储氢环节中都表现出了优良的适用性。特别是杂环芳烃可逆储放氢系统,是目前较为理想的有机液体储氢材料。而该储氢系统的催化脱氢过程存在着脱氢效率低、催化剂贵金属用量高等问题,制约着其实际应用。
在前序研究的基础上,设计了一种简便温和的一锅法共还原制备得到了一系列不同配比的还原氧化石墨烯负载的双金属PdCu/rGO合金催化剂,其中Pd1.2Cu/rGO催化剂首次在453K下达到了脱氢终产物杂环芳烃100%的选择性,同时贵金属Pd的用量相对于该反应中常用的商业催化剂和其他课题组已报道的催化剂降低了60%以上。同时,本研究系统分析了共还原温度和金属负载量的变化对脱氢反应动力学的影响,结合一系列催化剂的表征,解释了双金属PdCu结构与性能间的构效关系。通过关联双金属PdCu纳米粒子的配比和电子结构,验证了不同配比催化剂的活性规律。这一工作首次建立了杂环芳烃的宏观脱氢性能与活性组分微观电子结构间的关联,为相关有机液体储氢材料脱氢催化剂的设计提供了重要的指导和研究思路。目前正在推进该技术的工程放大与产业应用。
My research target is as follows:
(1) To design the new catalysts for the hydrogenation and dehydrogenation reactions with the performance of high activity, selectivity and stability.
(2) To apply the ionic liquid in the hydrogenation/dehydrogenation process as co-catalyst or “green” solvent.
(3) To simulate and calculate the geometric configuration and energy of molecule, the transition state, activation energy and the reaction mechanism.
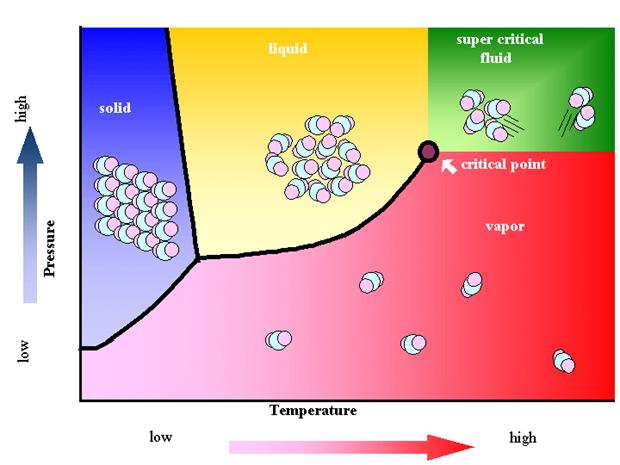
2. Biomass updating technology 生物质能
► Producing biodiesel (fatty acid methyl esters) with supercritical methanol
(Cooperator: Prof. Motonobu Goto, Nagoya Univ. Japan)
利用超临界甲醇制备生物柴油(脂肪酸甲酯)
(合作方:名古屋大学化工系後藤元信教授)
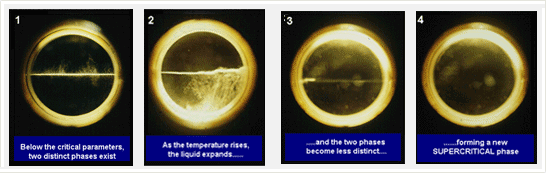
3. Supercritical fluids in combination with high electric field 超临界流体与高压电场的复合技术
► High Pressure gases - assisted Electrospinning for Producing Polymer Nanofibers aiming at Hydrogen Storage
(supported by Alexander von Humboldt foundation, Germany, Cooperator: Prof. Wolfgang Arlt, Erlangen-Nuremberg Univ., Germany)
高压流体辅助静电纺丝制备拓扑结构的微纳米纤维及其在储氢领域的应用
(资助方:德国洪堡基金会;合作方:德国埃尔兰根-纽伦堡大学,Wolfgang Arlt 教授)
► Generation and Application of Non-thermal Plasma in Supercritical CO2
(supported by Japan Society for the Promotion of Science (JSPS) and 21st century Center of Excellence (COE) project of ‘Pulse Power Science and Its Application’, Japan)
在超临界CO2中非热等离子体的制备与应用
(资助方:日本学术振兴会(JSPS)、21世纪COE项目“脉冲科学与应用”)
4. Fundamental research on phase equilibrium 流体相平衡基础研究
► Binary and ternary phase behavior of biodiesel and tocopherols in supercritical methanol
► Binary and ternary phase behavior of biodiesel and tocopherols in supercritical methanol
(Cooperators: Prof. Motonobu Goto, Nagoya Univ.; Dr. Yusuke Shimoyama and Prof. Yoshio Iwai, Kyushu Univ. Japan)
生物柴油、生育酚在超临界甲醇中的二元、三元相平衡
(合作方:日本名古屋大学後藤元信教授、九州大学岩井芳夫教授)
► Binary and ternary phase behavior of methyl oleate and tocopherols in supercritical CO2
(Cooperators: Prof. Xiaolin Ding, Jiangnan Univ., China; Prof. Zhi Yun, Nanjing Univ. of Tech., China; and Prof. Motonobu Goto, Nagoya Univ. Japan)
18碳生物柴油、生育酚与超临界CO2的二元、三元相平衡
(合作方:江南大学丁霄霖教授、南京工业大学云志教授、名古屋大学後藤元信教授)
► Measurement and calculation for the breakdown voltage under supercritical fluids
(Cooperators: Prof. Motonobu Goto, Nagoya Univ., Japan; Prof. Chaohai Zhang, Harbin Inst. of Tech., China)
超临界流体中击穿电压的测量与模拟计算
5. Processing of natural materials 天然产物精制过程
► Refining essential oil with countercurrent supercritical CO2 fractionation
(in cooperation with Shiono Flavor Co. Ltd., Osaka Japan)
超临界CO2逆流萃取分馏精制高附加值生物活性组分
(合作方:日本大阪盐野香精株式会社)
► Producing Natural Tocopherols (vitamin E) and Sterols by supercritical CO2 fractionation
(in cooperation with Prof. Xiaolin Ding, Jiangnan Univ., China; Prof. Motonobu Goto, Kumamoto Univ., Japan; and Wuhan Kaidi Fine Chemical Co. Ltd., China)
基于超临界流体的生物柴油、天然生育酚(维生素E)与甾醇制备工艺及其产业化示范
(合作方:武汉凯迪精细化工有限公司、江南大学、熊本大学)
6. The upgrading of heavy oils and the key technology based on supercritical fluid
超临界流体应用于重质油轻质化反应
World crude oil is becoming heavy seriously. The viscosity is increasing and the content of heteroatoms is higher. The heavy oils are putting forward higher requirements for the conventional processing technology. Supercritical methanol has excellent transport property and reactivity. Supercritical methanol has a good solubility to the macromolecular organics, as polymers and has been widely applied in the extraction, materials processing and plastic degradation, etc. In the chemical reaction study, supercritical methanol is used for organic synthesis and biomass processing. In the supercritical methanol, heavy oils can be modified without catalysts and external hydrogen. The process can promote the yield of light fractions and suppress the coke formation. Supercritical methanol has good applicability to different kinds of crude oil.
Research contents: to study the dynamic properties of heavy oils cracking in supercritical methanol, explore the mechanism of methanol during the cracking process.


 (创新港)
(创新港)


