Guanghu Tong’s work was published on Chem - 首页
Congratulation!
Guanghu Tong’s work on Total Synthesis of (±)Prostratin was published on Chem(IF= 14.104)
Title: Total Synthesis of (±)Prostratin
Authors: Guanghu Tong, Zhi Liu, Pengfei Li*
Links: https://doi.org/10.1016/j.chempr.2018.10.002
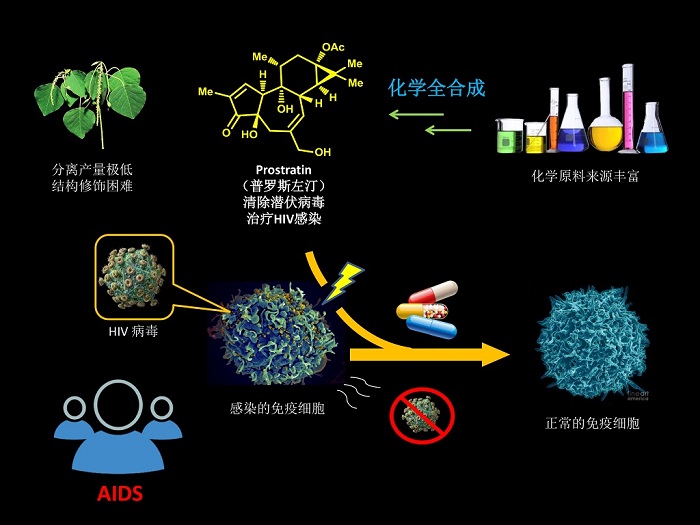
Professor Li Pengfei of the Frontier Institute of Science and Technology of Xi'an Jiaotong University has been engaged in the research on the total synthesis of complex natural products with important physiological activities and the catalytic reactions of transition metals. Guanghu Tong took cyclopentadiene as the starting material. Through epoxidation ring opening reaction, photosinglet oxygen oxidative dearomatization, palladium catalyzed coupling reaction, vinyl addition reaction promoted by large site hindrance Lewis acid, solvent and acid controlled stereoselectivity to construct cyclopropane and olefin complex, decomposition, cyclization, selective oxidation and other key reactions, the synthesis of Prostratin was completed succinctly and efficiently. The synthesis strategy of modularized and rapid construction of tricyclic core skeleton and stereoselective construction of multiple continuous chiral centers will be of great significance to the synthesis of related diterpene natural products and pharmaceutical chemistry.
西安交大前沿院李鹏飞教授课题组一直致力于具有重要生理活性的复杂天然产物的全合成和过渡金属催化反应的相关研究。课题组佟广虎博士生经过五年的不懈努力,以易得的环戊二烯为起始原料,经过环氧化开环反应、光照单线态氧氧化去芳化、钯催化偶联反应、大位阻路易斯酸促进下的乙烯基加成反应、溶剂和酸控制的立体选择性构建环丙烷、烯烃复分解关环反应、选择性氧化等关键反应,简洁高效地完成了巴豆烷二萜普罗斯左汀(Prostratin)的全合成。其中模块化快速构筑三环核心骨架以及立体选择性构建多个连续手性中心的合成策略,将对未来相关二萜天然产物的合成研究以及药物化学研究具有重要意义。
-
2023
06-29
-
2023
06-21
-
2023
06-01
-
2023
05-15
-
2022
10-05
-
2019
09-29

 (创新港)
(创新港)


