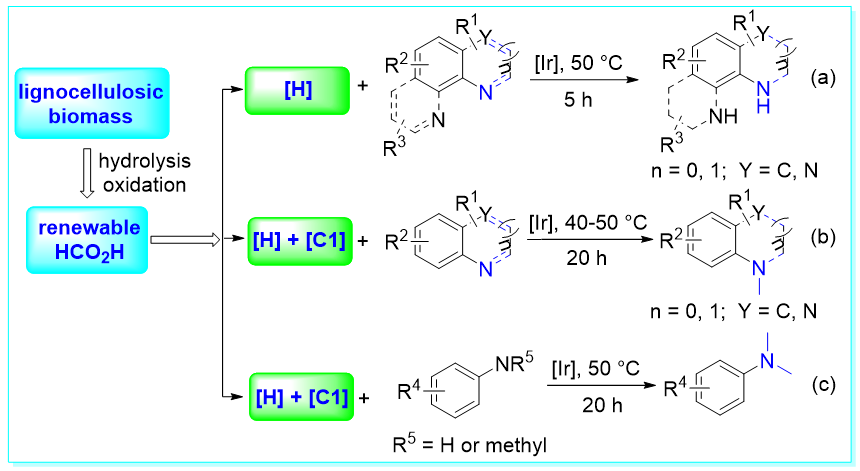
49. Selective Transformation of Biomass and the Derivatives for Aryl Compounds and Hydrogen via Visible-Light-Induced Radicals, Wen-Min Zhang, Wen-Ting Niu, Fang-Fang Tan, and Yang Li*, Acc. Chem. Res., 2025, 58: acs.accounts. 5c00038.
https://doi.org/10.1021/acs.accounts.5c00038

48. Efficient Water Reforming of Biomass to H2 via Well-Organized Redox-Neutral Cleavage of C−C, O−H and C−H Bonds, Wen-Min Zhang and Yang Li*, Angew. Chem. Int. Ed. 2024, e202416867.
https://doi.org/10.1002/anie.202416867

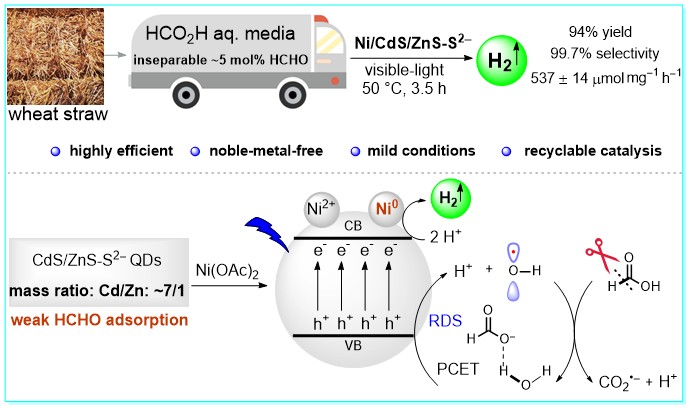
47. Efficient H2 Production from Biomass-Based HCO2H by Cooperation of Quantum Dots Photocatalysts with Weak HCHO Adsorption and In Situ Generated Ni0, Wen-Ting Niu, Wanghui Zhao, Kai-Wen Feng, Fu-Jia Tang, Tao Wang, Kaixuan Wang, Shaohua Shen, and Yang Li*, ACS Catal. 2024, 14, 10194–10203.
https://doi.org/10.1021/acscatal.4c01708
46. Organic charge-transfer complex induces chemoselective decarboxylation to aryl radicals for general functionalization, Chun-Hong Hu, Yueqian Sang, Ya-Wei Yang, Wen-Wen Li, Hui-Lin Wang, ZiYing Zhang, Chen Ye*, Li-Zhu Wu, Xiao-Song Xue*, and Yang Li*, Chem 2023, 9, 2997–3012.
https://doi.org/10.1016/j.chempr.2023.06.022

45. Photoredox and Vanadate Cocatalyzed Hydrolysis of Aryl Ethers at Ambient Temperature, Rong-Gui Hu, Yueqian Sang, Fang-Fang Tan, Yuan-Li Sun, Xiao-Song Xue*, and Yang Li*, ACS Catal. 2023, 13, 9264–9273.(Hightlighted by Advances in Engineering)
https://doi.org/10.1021/acscatal.3c02301

44. Hydrogen Production from Formic Acid by in situ Generated Ni/CdS Photocatalytic System under Visible Light Irradiation, Kai-Wen Feng and Yang Li*, ChemSusChem 2023, 16, e202202250.
https://doi.org/10.1002/cssc.202202250

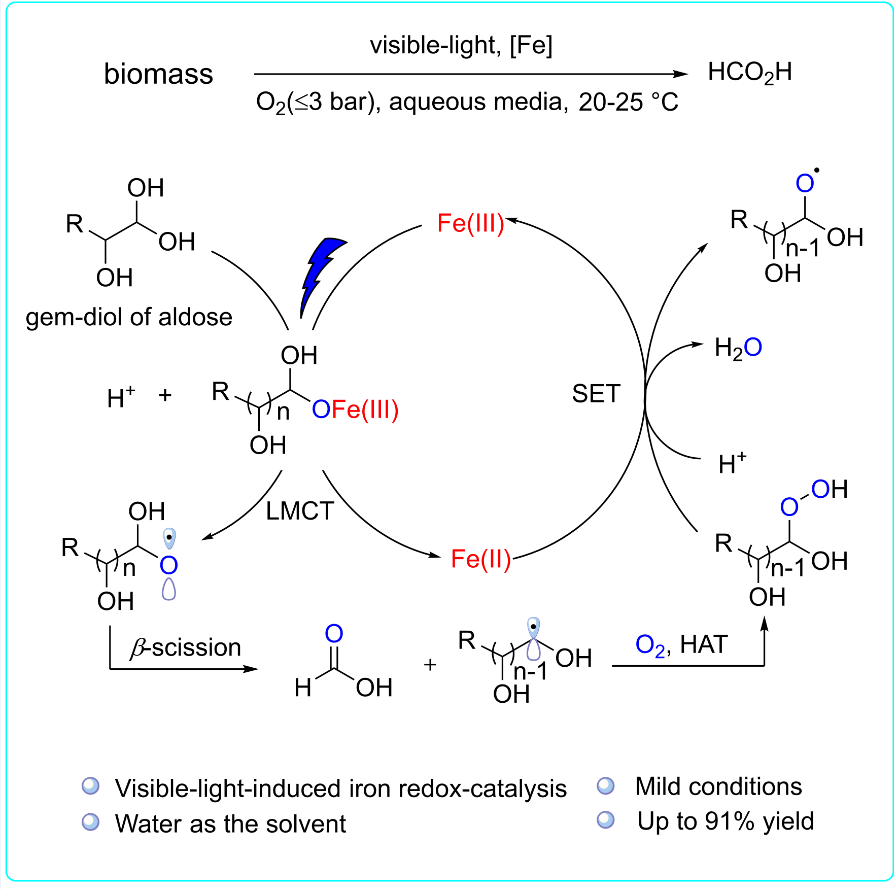
43. Visible-Light-Induced Iron Redox-Catalyzed Selective Transformation of Biomass into Formic Acid, Wen-Min Zhang, Kai-Wen Feng, Rong-Gui Hu, Yan-Jun Guo, and Yang Li*, Chem 2023, 9, 430–442.
https://doi.org/10.1016/j.chempr.2022.10.011
42. Visible-Light Photoredox-Catalyzed Decarboxylation of α‑Oxo Carboxylic Acids to C1-Deuterated Aldehydes and Aldehydes, Chun-Hong Hu and Yang Li*, J. Org. Chem. 2023, 88, 6401–6406. (Highlighted by https://www.organic-chemistry.org/Highlights/2024/22January.shtm)
https://doi.org/10.1021/acs.joc.2c02299

41. Visible-Light Photoredox-Catalyzed Hydrodecarboxylation and Deuterodecarboxylation of Fatty Acid, Yuan-Li Sun, Fang-Fang Tan, Rong-Gui Hu, Chun-Hong Hu, and Yang Li*, Chin. J. Chem. 2022, 40, 1903–1908.
https://doi.org/10.1002/cjoc.202200143

40. Utilization of renewable formic acid from lignocellulosic biomass for the selective hydrogenation and/or N-methylation, Chao-Zheng Zhou, Yu-Rou Zhao, Fang-Fang Tan, Yan-Jun Guo, and Yang Li*, ChemCatChem 2021, 13, 4724–4728.
https://doi.org/10.1002/cctc.202101099
39. Practical DMSO-promoted selective hydrolysis-oxidation of lignocellulosic biomass to formic acid attributed to hydrogen bonds, Yan-Jun Guo, Shi-Jun Li, Yuan-Li Sun, Lei Wang, Wen-Min Zhang, Ping Zhang, Yu Lan* and Yang Li*, Green Chem. 2021, 23, 7041–7052.
https://doi.org/10.1039/D1GC02265B

38. Formic Acid Disproportionation into Formaldehyde Trigged by Vanadium Complexes With Iridium Catalysis Under Mild Conditions in the N-methylation, Chao-zheng Zhou, Yu-Rou Zhao, Yan-Jun Guo, Ping Zhang and Yang Li*, Green Chem. 2021, 23, 2918–2924.
https://doi.org/10.1039/D1GC00275A

37. Visible-light photoredox-catalyzed C-O bond cleavage of diaryl ethers by acridinium photocatalysts at room temperature, Fang-Fang Tan, Xiao-Ya He, Wan-Fa Tian, and Yang Li*, Nat. Commun. 2020, 11, 6126. (Highlighted by Synfacts 2021; 17(03): 0290. DOI: 10.1055/s-0040-1720316)
https://doi.org/10.1038/s41467-020-19944-x

36. Across the Board: Yang Li on Visible-Light Photoredox Catalysis, Yang Li*, ChemSusChem 2020, 13, 3937–3939.
https://doi.org/10.1002/cssc.202001499

35. Visible-Light Photoredox-Catalyzed Decarboxylative Alkylation of Heteroarenes Using Carboxylic Acids with Hydrogen Release, Wan-Fa Tian, Chun-Hong Hu, Ke-Han He, Xiao-Ya He, and Yang Li*, Org. Lett. 2019, 21, 6930–6935.
https://doi.org/10.1021/acs.orglett.9b02539

34. Utilization of Hydrogen Source from Renewable Lignocellulosic Biomass for Hydrogenation of Nitroarenes, Fang-Fang Tan, Kai-Li Tang, Ping Zhang, Yan-Jun Guo, Meng-Nan Qu, and Yang Li*, ChemCatChem 2019, 11, 4189–4195.
https://doi.org/10.1002/cctc.201900087

33. Streamlined Hydrogen Production from Biomass, Ping Zhang, Yan-Jun Guo, Jianbin Chen, Yu-Rou Zhao, Jun Chang, Henrik Junge, Matthias Beller*, and Yang Li*, Nat. Catal. 2018, 1, 332–338. (Highlighted by Chemistry World www.chemistryworld.com/news/fuelling-the-hydrogen-economy-with-straw-and-old-newspapers/3008978.article and reported by Chemical Science Department of National Natural ScienceFund Committee. chem.nsfc.gov.cn/Show.aspx).
https://doi.org/10.1038/s41929-018-0062-0


32. Visible-light Photoredox-Catalyzed Iminyl Radical Formation by N-H Cleavage with Hydrogen Release and Its Application in Synthesis of Isoquinolines, Wan-Fa Tian, Dang-Po Wang, Shao-Feng Wang, Ke-Han He, Xiao-Ping Cao, and Yang Li*, Org. Lett. 2018, 20, 1421−1425.
https://doi.org/10.1021/acs.orglett.8b00193
31. An Efficient Aryl Migration from an Aryl Ether to Ester Ether by Visible-Light Photoredox Catalysis, Shao-Feng Wang, Xiao-Ping Cao*, and Yang Li*, Angew. Chem. Int. Ed. 2017, 56, 13809–13813. (Highlighted by Synfacts, 2017, 13, 1317).
https://doi.org/10.1002/anie.201706597

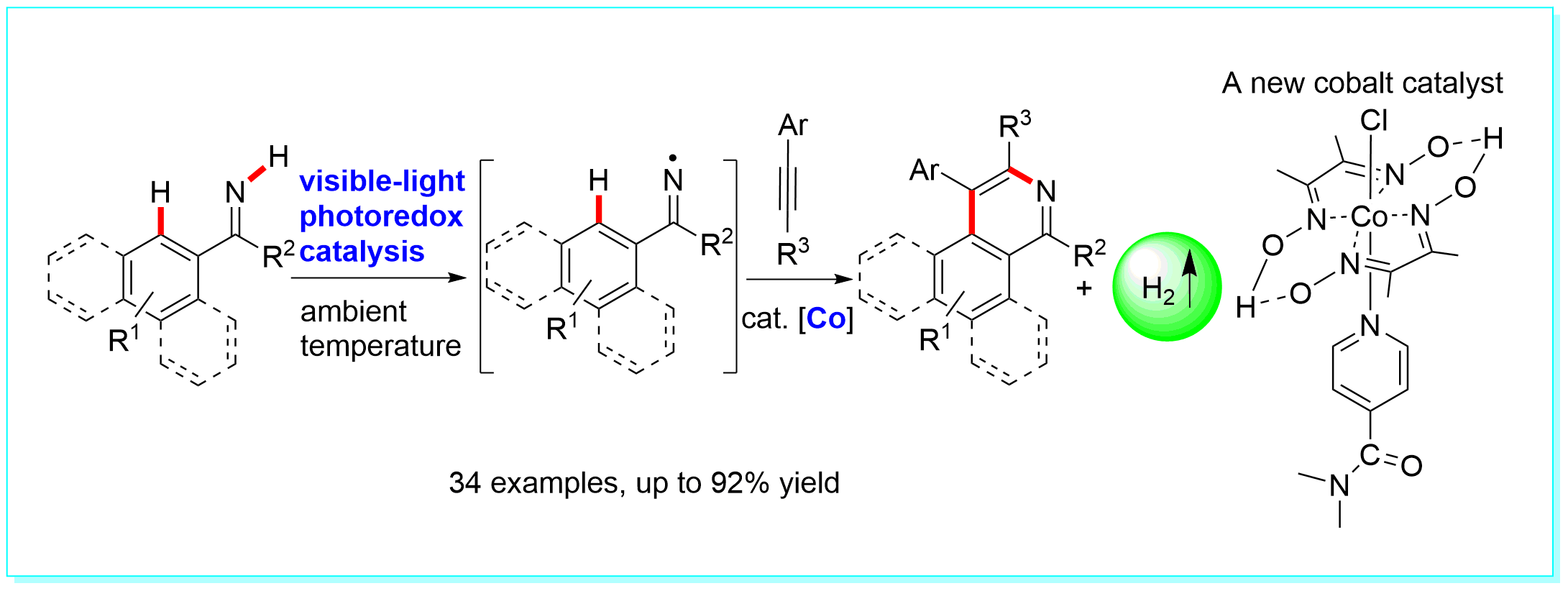
30. Acceptorless Dehydrogenation of N-Heterocycles by Merging Visible-Light Photoredox Catalysis and Cobalt Catalysis, Ke-Han He, Fang-Fang Tan, Chao-Zheng Zhou, Gui-Jiang Zhou, Xiao-Long Yang, and Yang Li*, Angew. Chem. Int. Ed. 2017, 56, 3080–3084. (Highlighted by Angew. Chem. Int. Ed. 2017, 56, 7716–7718, Chin. J. Org. Chem. 2017, 37, 1051).
https://doi.org/10.1002/anie.201612486

29. Redox-Divergent Hydrogen-Retentive or Hydrogen-Releasing Synthesis of 3,4-Dihydroisoquinolines or Isoquinolines, Ke-Han He, Wei-Dong Zhang, Ming-Yu Yang, Kai-Li Tang, Mengnan Qu, You-Song Ding, and Yang Li*, Org. Lett. 2016, 18, 2840–2843.
https://doi.org/10.1021/acs.orglett.6b01091

28. Rhodium-catalyzed Inert Chemical Bonds Transformation and Related Mechanism Investigation, Yang Li*, Zhang-Jie Shi*, Sci. Sin. Chem., 2016, 46, 579–587. (Invited Paper).
https://doi.org/10.1360/N032015-00224
26. Oxidant-Free Dehydrogenative Coupling Reactions via Hydrogen Evolution, Ke-Han He, Yang Li*, ChemSusChem 2014, 7, 2788–2790.
https://doi.org/10.1002/cssc.201402606

Before Joining FIST-XJTU
27 “Group Exchange between Ketones and Carboxylic Acids through Directing Group Assisted Rh-catalyzed Reorganization of Carbon Skeletons ”, Lei, Z.-Q.; Pan, F.; Li, H.; Li, Y.; Zhang,X.-S.; Chen, K.; Wang, X.; Li, Y.-X.; Sun, J.; Shi, Z.-J. J. Am. Chem. Soc. 2015, 137, 5012-5020.
25 “Efficient and Selective Hydrogen Generation from Bioethanol using Ruthenium Pincer-type Complexes”, Sponholz, P.; Mellmann, D.; Cordes, C.; Alsabeh, P. G.; Li, B.; Li, Y.; Nielsen, M.; Junge, H.; Dixneuf , P.; Beller M. ChemSusChem, 2014, 7, 2419-2422.
24 “Selective Ruthenium-catalyzed Methylation of 2-Arylethanols using Methanol as C1 Feedstock”, Li, Y.; Li, H.;Junge, H.; Beller, M. Chem. Commun. 2014, 50, 14991.

23 “Ruthenium-catalyzed Hydrogen Generation from Glycerol and Selective Synthesis of Lactic Acid”, Li, Y.; Nielsen, M.; Li, B.; Dixneuf, P. H.; Junge, H.; Beller, M. Green Chem, 2015, 17, 193.

22 “A Molecular-defined Iridium Catalyst for Direct Hydrogen Production from Monosaccharides, Disaccharide, Cellulose and Lignocellulose”, Li, Y.; Sponholz, P.; Nielsen, M.;Junge, H.; Beller, M. ChemSusChem, 2015, 8, 804.
The video for the reaction procedure (It is easier played by Google Explorer, Firefox Explorer):
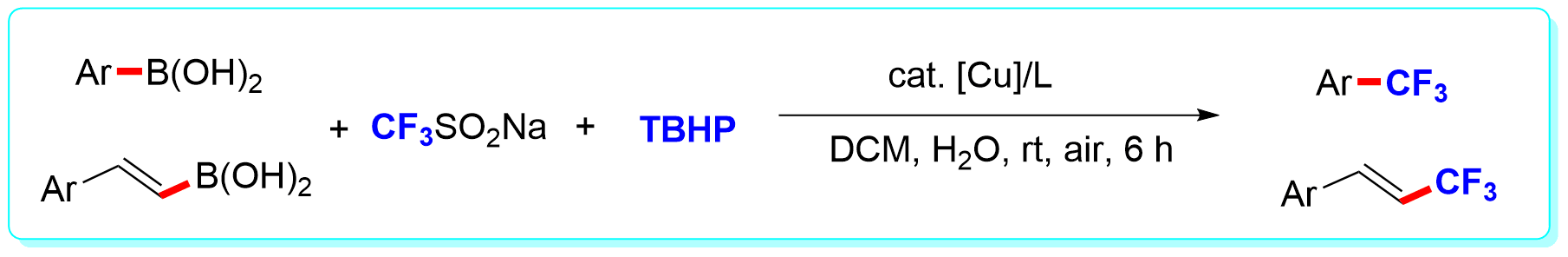
21 “Copper-catalyzed Trifluoromethylation of Aryl- and Vinylboronic Acids with Generation of CF3-radicals”, Li, Y.; Wu, L.; Neumann, H.; Beller, M. Chem. Commun. 2013, 49, 2628 (ESI highly cited paper).
20 “Olefinic C–H Bond Addition to Aryl Aldehyde and Its N-Sulfonylimine via Rh Catalysis” Li, Y.; Zhang, X.-S.; Zhu, Q.-L.; Shi, Z.-J. Org. Lett.2012, 14, 4498–4501.

19 “Mechanistic Insight into the Regioselective Palladation of Indole Derivatives: Tetranuclear Indolyl Palladacycles with High C2−Pd or C3−Pd Bond Selectivity”, Li, Y.; Wang, W.-H.; He, K.-H.; Shi, Z.-J. Organometallics 2012, 31, 4397.

18 “Mechanistic Understanding of Rh-Catalyzed N-Sulfonylaldimine Insertion to Aryl C-H Bonds”, Li, Y.; Zhang, X.-S.; Li, H.; Wang, W.-H.; Chen, K.; Li, B.-J.; Shi, Z.-J. Chem. Sci. 2012, 3, 1634 (ESI highly cited paper).
The video for the reaction mechanism (It is easier played by Google Explorer,Firefox Explorer):
17 “N-Directing Group Assisted Rhodium-Catalyzed Aryl C-H Addition to Aryl Aldehydes”, Li, Y.; Zhang, X.-S.; Chen, K.; He, K.-H.; Pan, F.; Li, B.-J.; Shi, Z.-J. Org. Lett. 2012, 14, 636 (ESI highly cited paper).

16 “Rhodium-Catalyzed Direct Addition of Aryl C-H Bonds toN-Sulfonyl Aldimines”, Li, Y.; Li, B.-J.; Wang, W.-H.; Huang, W.-P.; Zhang, X.-S.; Chen. K.; Shi, Z.-J. Angew. Chem. Int. Ed. 2011, 50, 2115 (ESI highly cited paper).

15 “Oxidative Dimerization of N-Protected and Free Indole Derivatives toward 3,3´-Biindoles viaPd-catalyzed Direct C–H Transformations”, Li, Y.; Wang, W.-H.; Yang, S.-D.; Li, B.-J.; Feng, C.; Shi, Z.-J. Chem. Commun. 2010, 46, 4553.

14 “Total Synthesis and Correct Absolute Configuration of Malyngamide U”, Li, Y.; Feng, J.-P.; Wang, W.- H.; Chen, J.; Cao, X.-P. J. Org. Chem. 2007,72, 2344 (most-accessed article for the full year of 2007).

13 “Rh-Catalyzed C-C Cleavage of Benzyl/Allylic Alcohols to Produce Benzyl/Allylic Amines or Other Alcohols by Nucleophilic Addition of Intermediate Rhodacycles to Aldehydes and Imines ”, Zhang, X.-S.; Li, Y.; Li, H.; Chen, K; Lei, Z.-Q.; Shi, Z.-J. Chem. Eur. J. 2012,18, 16214.
12 “Reductive Cleavage of the Csp2-Csp3 Bond of Secondary Benzyl Alcohols: Rhodium Catalysis Directed by N-Containing Groups”, Chen, K.; Li, H.; Lei, Z.-Q.; Li, Y.; Ye, W.-H.; Zhang, L.-S.; Sun, J.; Shi, Z.-J. Angew. Chem. Int. Ed. 2012,51, 9851.
11 “Direct Oxidative Arylation via Rhodium-Catalyzed C-C Bond Cleavage of Secondary Alcohols with Arylsilanes”, Chen, K.; Li, H.; Li, Y.; Zhang, X.-S.; Lei, Z.-Q.; Shi, Z.-J. Chem. Sci. 2012, 3, 1645.
10 “Extrusion of CO from Aryl Ketones: Rhodium(I)-Catalyzed C-C Bond Cleavage Directed by a Pyridine Group”, Lei, Z.-Q.; Li, H.; Li, Y.; Zhang, X.-S.; Chen, K.; Wang, X.; Sun, J.; Shi, Z.-J. Angew. Chem. Int. Ed. 2012,51, 2690.
9 “Pyridinyl Directed Alkenylation with Olefins via Rh(III)-Catalyzed C–C Bond Cleavage of Secondary Arylmethanols”, Li; H.; Li, Y.; Zhang, X.-S.; Chen, K.; Wang, X.; Shi, Z.-J. .J. Am. Chem. Soc. 2011, 133, 15244.
8 “An Improved Asymmetric Synthesis of Malyngamide U and Its 2΄-Epimer”, Feng, J.-P.; Shi, Z.-F.; Li, Y.; Zhang, J.-T.; Qi, X.-L.; Chen, J.; Cao, X.-P. J. Org. Chem. 2008,73, 6873.
7 “A Stereoselective Synthesis of (4E,7S)-(–)-7-Methoxydodec-4-enoic Acid”, Li, Y.; Chen, J.; Cao, X.-P. Synthesis 2006, 320.
6 “First Stereoselective Synthesis of Serinol-derived Malyngamidesand Their 1΄-epi-isomers”, Chen, J.; Li, Y.; Cao, X.-P. Tetrahedron: Asymmetry 2006, 17, 933.
5 “The First Total Synthesis of Phebaclavin A and C”, Zhang, Y.; Liu, D.; Li, Y.; Cao, X.-P. Chin. J. Chem. 2005, 23, 1453.
4 “Stereoselective Synthesis of Naturally Occurring Unsaturated Amide Alkaloids by a Modified Ramberg-Bäcklund Reaction”, Li, Y.; Zhang, Y.; Huang, Z.; Cao, X.-P.; Gao, K. Can. J. Chem. 2004, 82, 622.
3 “Stereoselective Synthesis of Piperamide Alkaloids by a Modified Ramberg-Bäcklund Reaction”, Li, Y.; Zhang, Y.; Wang, X.-L.; Huang, Z.; Cao, X.-P. Chin. Chem. Lett.2004, 15, 631.
2 “Stereoselective Synthesis of (Z)-5-(Trideca-4-enyl) Resorcinol and Gibbilimbols A–D”, Zhou, L.; Li, Y.; Cao, X.-P. Chin. J. Chem. 2004, 22, 1344.
1 “A Novel Practical Reaction of α,β–Unsaturated Esters and Ketones with Lithium Aluminum Hydride”, Li, Y.; Wang, F.; Cao, X.-P. Acta Chimica Sinica 2003, 61, 279.





