祝贺硕士毕业生余宝柱水系锌离子电池CS@Ce-MnO2正极研究工作在 Journal of Colloid And Interface Science顺利发表! - 首页
近日硕士毕业生余宝柱基于多孔碳球材料基氧化锰正极的水系锌离子电池研究工作在Journal of Colloid And Interface Science顺利发表!
https://doi.org/10.1016/j.jcis.2023.10.009
Abstract
Aqueous zinc-ion batteries (ZIBs) have been considered one of the most promising flexible chemical power sources, because of their affordable cost, absolute security, and lightweight. However, the development of flexible aqueous ZIBs has been hindered by cathode materials due to their unsatisfied capacity, unstable structure, and ambiguous electrochemical energy storage mechanism. To address the above issues, a high-performance manganese cerium-doped dioxide-based core–shell hybrid structure cathode (CS@Ce-MnO2) has been successfully prepared via a facile low-temperature liquid-phase reaction strategy. Benefit from the delicately designed hierarchical carbon spheres core and cerium-doped manganese dioxide nanosheets shell structure, the capacity and stability of CS@Ce-MnO2 based flexible ZIBs has been dramatically improved, and the origin of the improved electrochemical performance and storage mechanism was demonstrated by electrochemical methods and ex-site x-ray diffraction (XRD) and scanning electron microscopy (SEM). The principal reason for the high reversible specific capacity is the plausible Zn2+ and H+co-insertion/extraction, while the porous structure of the carbon spheres contributes to the improved electron conduction and ion transport in the MnO2 matrix. This work provides a new opportunity for high-performance flexible aqueous zinc-ion batteries.
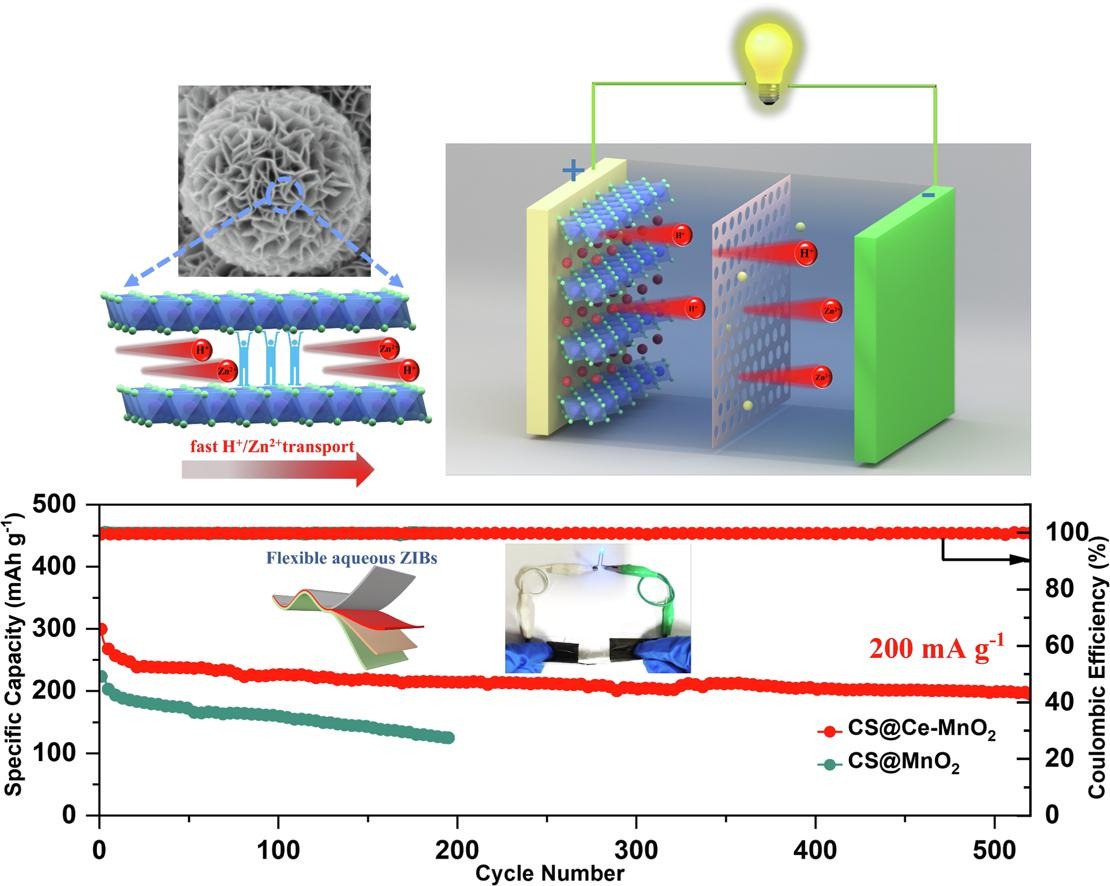
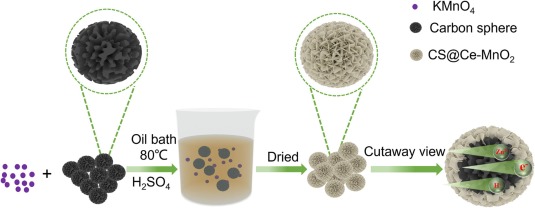
Fig. 1. Illustration of the fabrication process for CS@Ce-MnO2.

Fig. 2. Material characterization. SEM images of (a) carbon sphere, (b) CS@MnO2-5, (c) CS@Ce-MnO2-15, (d) CS@MnO2-30. (e-g) TEM images and the elemental mapping of CS@Ce-MnO2-15. (h) HRTEM image of CS@Ce-MnO2-15. (i) XRD patterns, and (j) The XPS spectrum of CS@MnO2-15 and CS@Ce-MnO2-15. (k) the Mn 2p XPS spectrum of CS@MnO2-15 and CS@Ce-MnO2-15.

Fig. 3. (a) The schematic diagram of flexible ZIBs. (b) The CV curve of MnO2 at a sweep speed of 0.1 mV s−1, CS@Ce-MnO2-15, CS@MnO2-15, and MnO2. (c) GCD curves at a current density of 200 mA g−1. (d) The CV curves of CS@Ce-MnO2-15 at different sweep speeds. (e) relationship between log (i) and log (v) plot at corresponding redox peaks. (f) Capacitance diffusion controls the contribution of charge to the scanning rate.

Fig. 4. (a) The charge–discharge curves of CS@Ce-MnO2-15 at different current densities. (b) The multiplier performance of the battery. (c) Ragone plot of CS@MnO2-15 and CS@Ce-MnO2-15. (d) The performance comparison of CS@Ce-MnO2-15 electrode material with other cathode materials. (e) Cycling performance of CS@MnO2-15 and CS@Ce-MnO2-15 electrodes at 100 mA g−1 current density. (f) GITT of CS@Ce-MnO2-15 and CS@MnO2-15 electrode. (g) Cycling performance of CS@Ce-MnO2-15 and CS@MnO2-15 at 200 mA g−1. (h) EIS of Ce-CS@MnO2 with different number of cycles. (i) Cycling performance of CS@Ce-MnO2-15 at 1000 mA g−1. (j) Comparison of electrochemical properties of CS@Ce-MnO2-15 cathode with other materials.
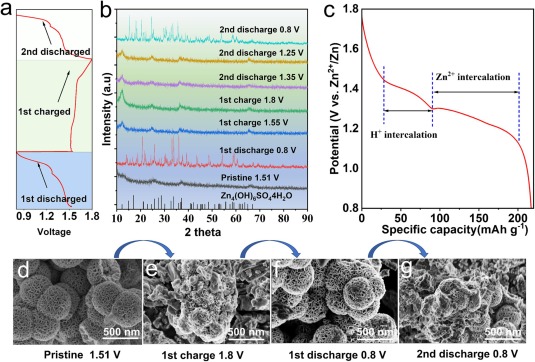
Fig. 5. (a) discharge/charge curves and (b) ex-situ XRD spectra of CS@Ce-MnO2-15 electrodes in different discharge/charge states. (c) discharge curve at 100 mA g−1 current density. SEM images of CS@Ce-MnO2-15 at different discharge/charge states; (d) Initial state, (e) The first discharge to 0.8 V, (f) The first charge up to 1.8 V, and (g) The Second discharge up to 0.8 V.
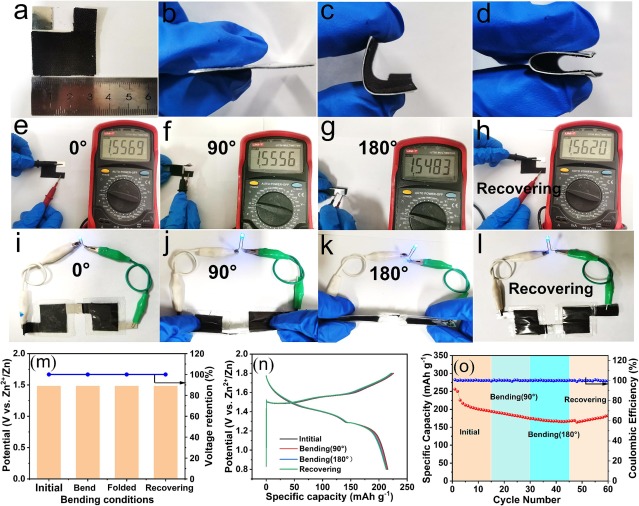
Fig. 6. (a) physical diagram of flexible zinc ion battery. (b-d) morphology of flexible zinc ion batteries under different bending angles. (e-h) open circuit voltage of the battery at different bending angles. (i-l) two cells in series battery lighting LEDs at different bending angles. (m) open circuit voltage retention of the battery at different bending states. (n) the GCD curve density of the prepared device at different bending states under current 100 mA g−1. (o) cycling stability of flexible batteries at 200 mA g−1 with different mechanical deformation.
-
2024
03-20
-
2024
03-11
-
2024
03-01
-
2023
11-21
-
2023
10-30
-
2023
10-17

 (创新港)
(创新港)


