祝贺翟文芳关于高性能ORR/OER双功能催化剂的研究论文被Applied Catalysis B期刊接收并上线! - 首页
祝贺翟文芳同学关于MOF基多金属单原子ORR/OER双功能催化剂的研究论文被催化类知名期刊Applied Catalysis B: Environmental接收并上线。
https://doi.org/10.1016/j.apcatb.2023.123438
Abstract
The fundamental challenge of trimetallic single-atoms catalysts stems from the difficulty in supporting the coexistence of different metals with their unique physicochemical features. Here, the trimetallic single-atoms electrocatalyst is constructed as a model system to explore electrocatalytic ORR/OER. The tri-metal (Fe, Co, and Ni) single-atoms loaded on nitrogen-carbon framework exhibits superior ORR/OER kinetics (E1/2 = 0.927 V, Ej=10 = 1.56 V). The flexible zinc-air batteries generate excellent power density and durability (62 mW cm−2 and 200 h at −15 °C, 291.2 mW cm−2 and 250 h at room temperature). Density functional calculations confirm that the trimetallic synergy effect outperform mono- and bimetallic effects, yielding adsorption strength close to 0. This paper describes an intuitionistic descriptor where ΔEOH* displays a volcano relationship with ORR overpotential, and establishes a generalized strategy for efficient bifunctional oxygen electrocatalyst while comprehensively understanding the structure-mechanism-activity relationship.
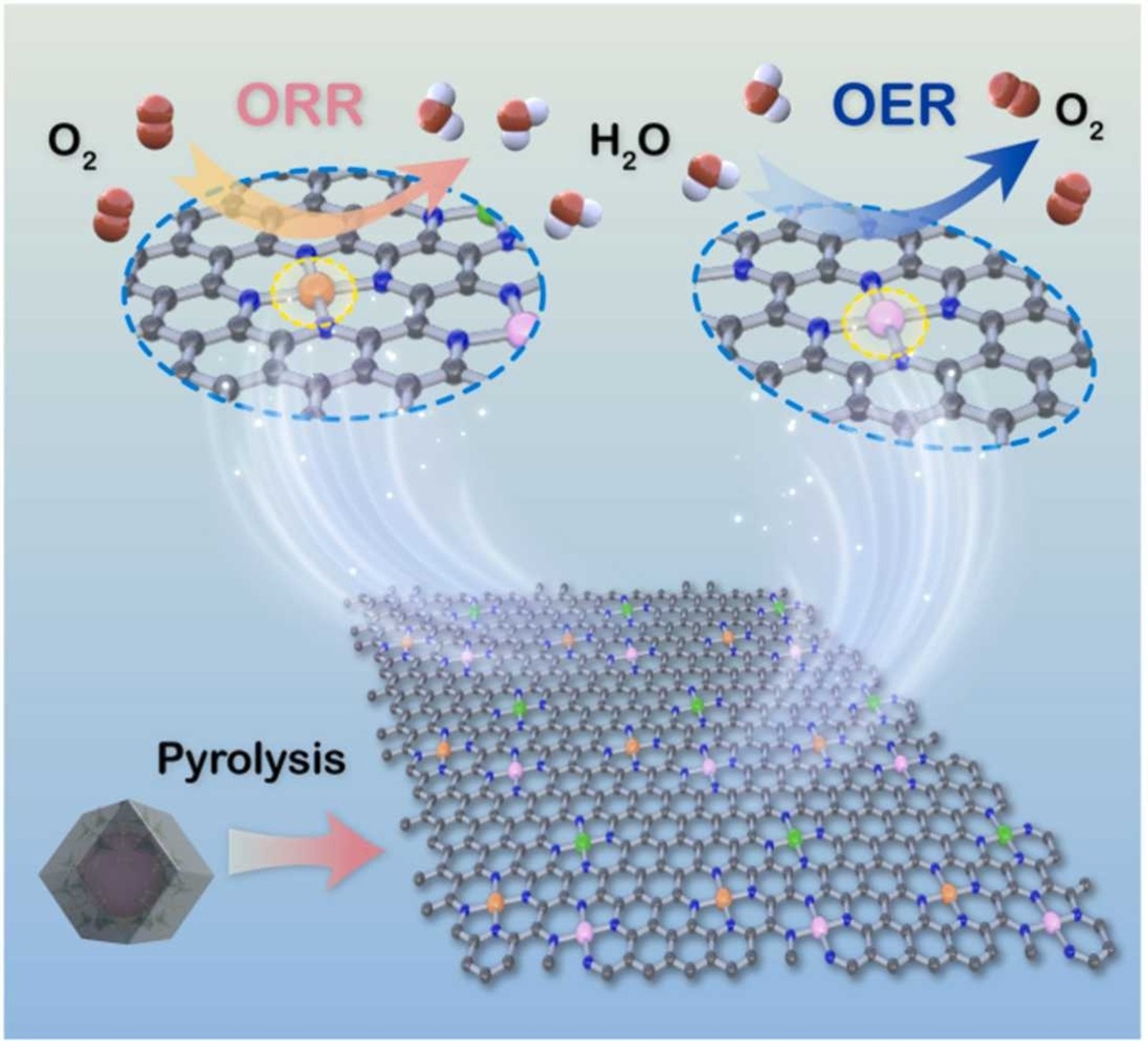
Trimetallic single-atoms nitrogen-carbon frameworks structures were fabricated by directly solvothermal method for efficient bifunctional oxygen electrocatalysts. Tri-metals (e.g. FeCoNi) are of covalent incorporation with nitrogen-carbon frameworks, presenting the excellent catalytic performances in oxygen reduction and oxygen evolution reactions in 0.1 M KOH electrolytes. FeCoNiNC-assembled flexible Zn-air batteries demonstrating low-temperature adaptability and highly power density and durable cycle stability.
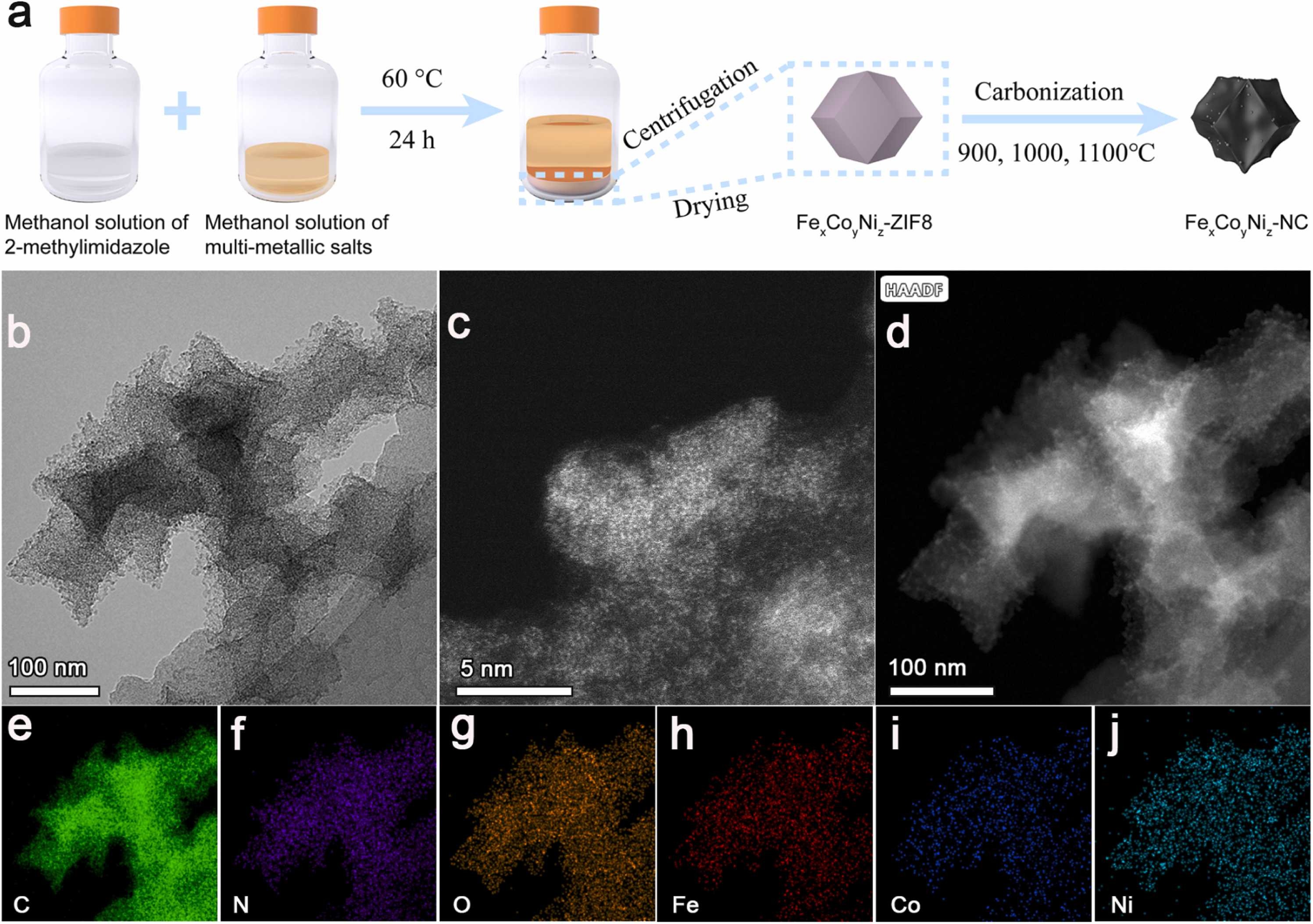
Fig. 1. a) Synthetic route of FexCoyNiz-NC. Morphological and structural information of Fe3%Co3%Ni9%-NC1000. b) TEM. c) AC-STEM image. d) AC HADDF-STEM, e-j) The corresponding elementary mapping images of C, N, O, Fe, Co, and Ni.
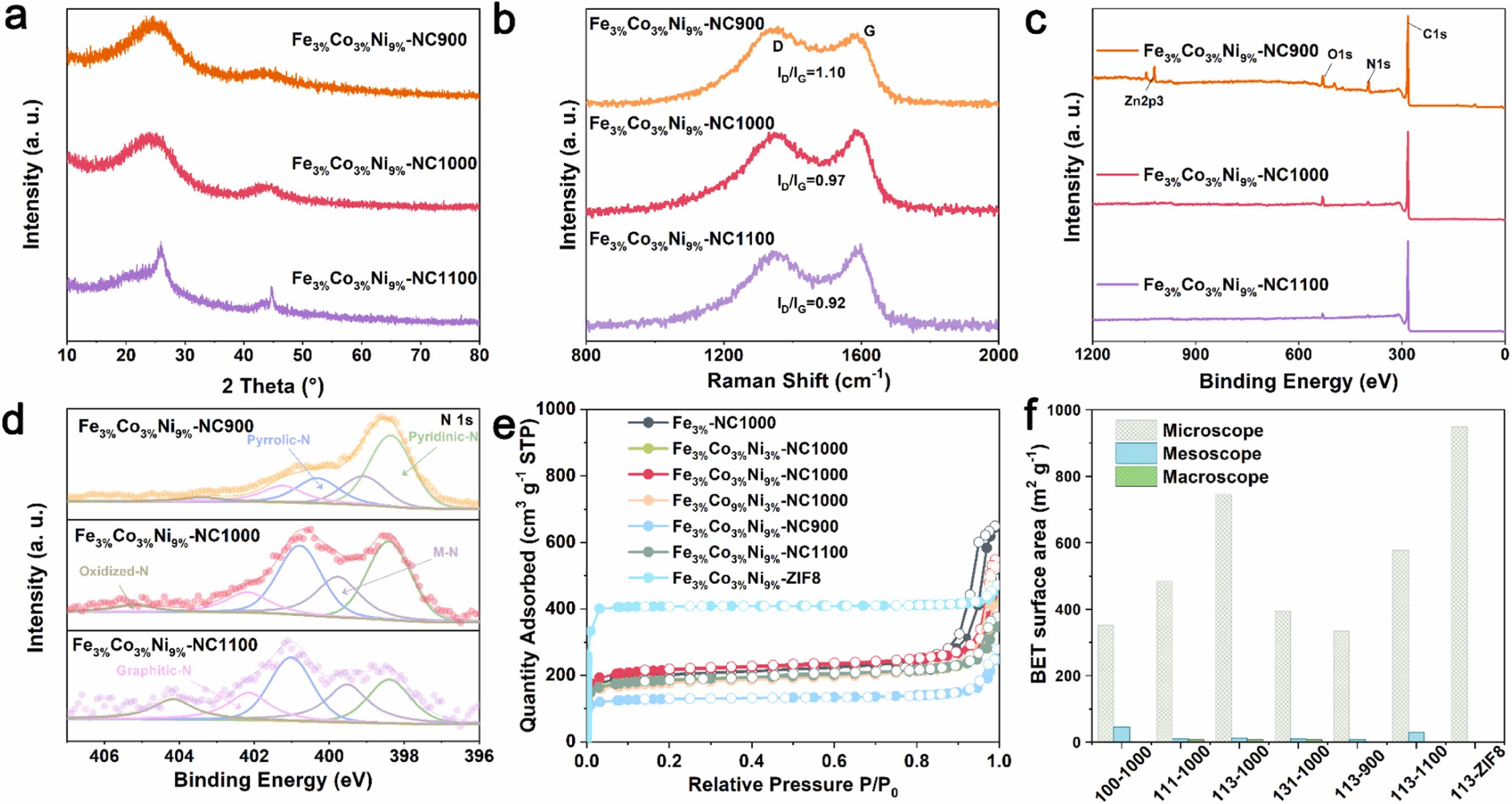
Fig. 2. a) XRD patterns. b) Raman spectrum. c) XPS survey. d) XPS N 1 s spectra. e) N2 adsorption/desorption isotherms. f) Pore distribution of catalysts.
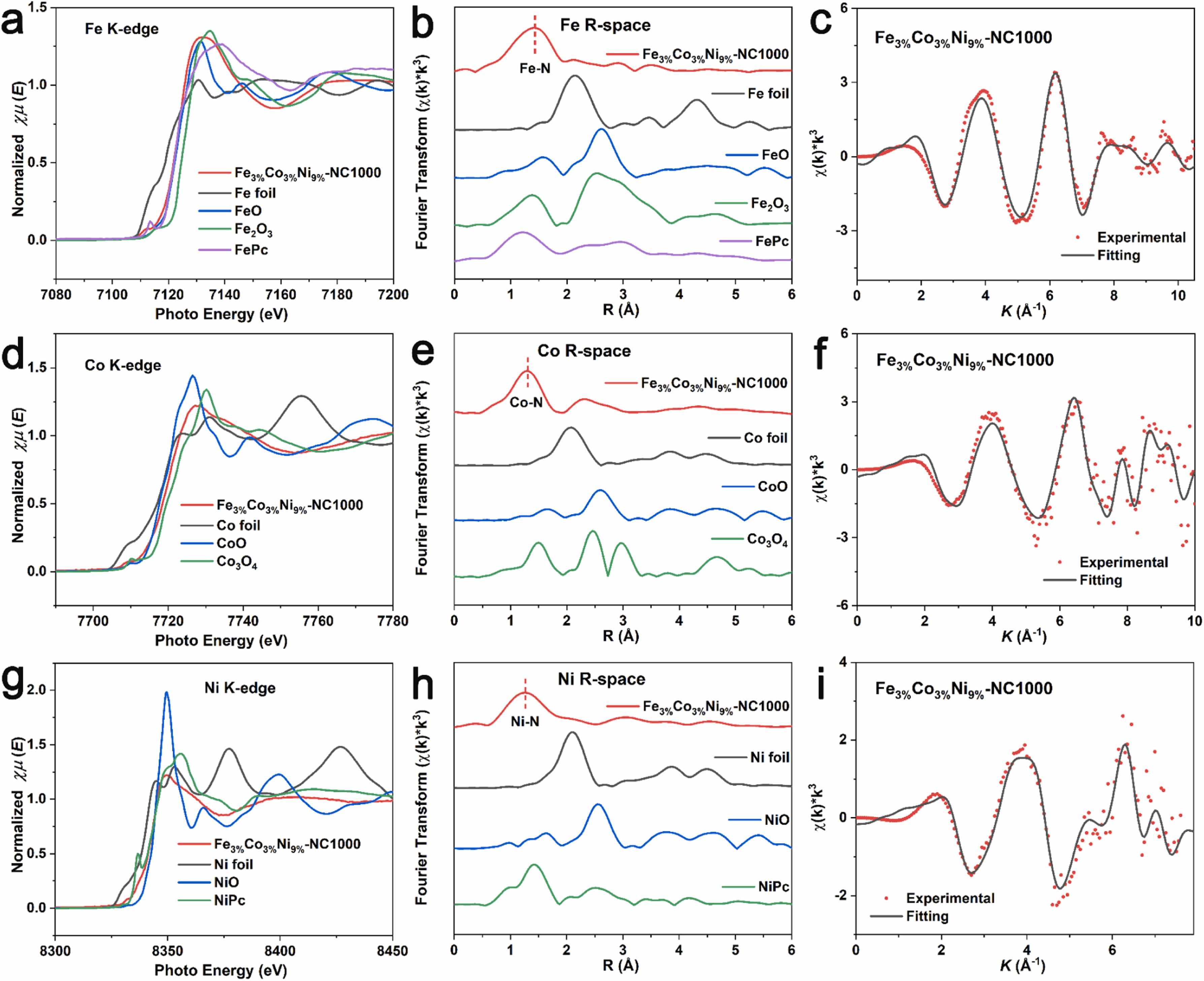
Fig. 3. a) Normalized Fe K-edge XANES spectra. b) k3-weighted χ(k) function of the EXAFS spectra. c) EXAFS fitting curve of Fe3%Co3%Ni9%-NC1000. d) Normalized Co K-edge XANES spectra. e) k3-weighted χ(k) function of the EXAFS spectra. f) EXAFS fitting curve of Fe3%Co3%Ni9%-NC1000. g) Normalized Ni K-edge XANES spectra. h) k3-weighted χ(k) function of the EXAFS spectra. i) EXAFS fitting curve of Fe3%Co3%Ni9%-NC1000.
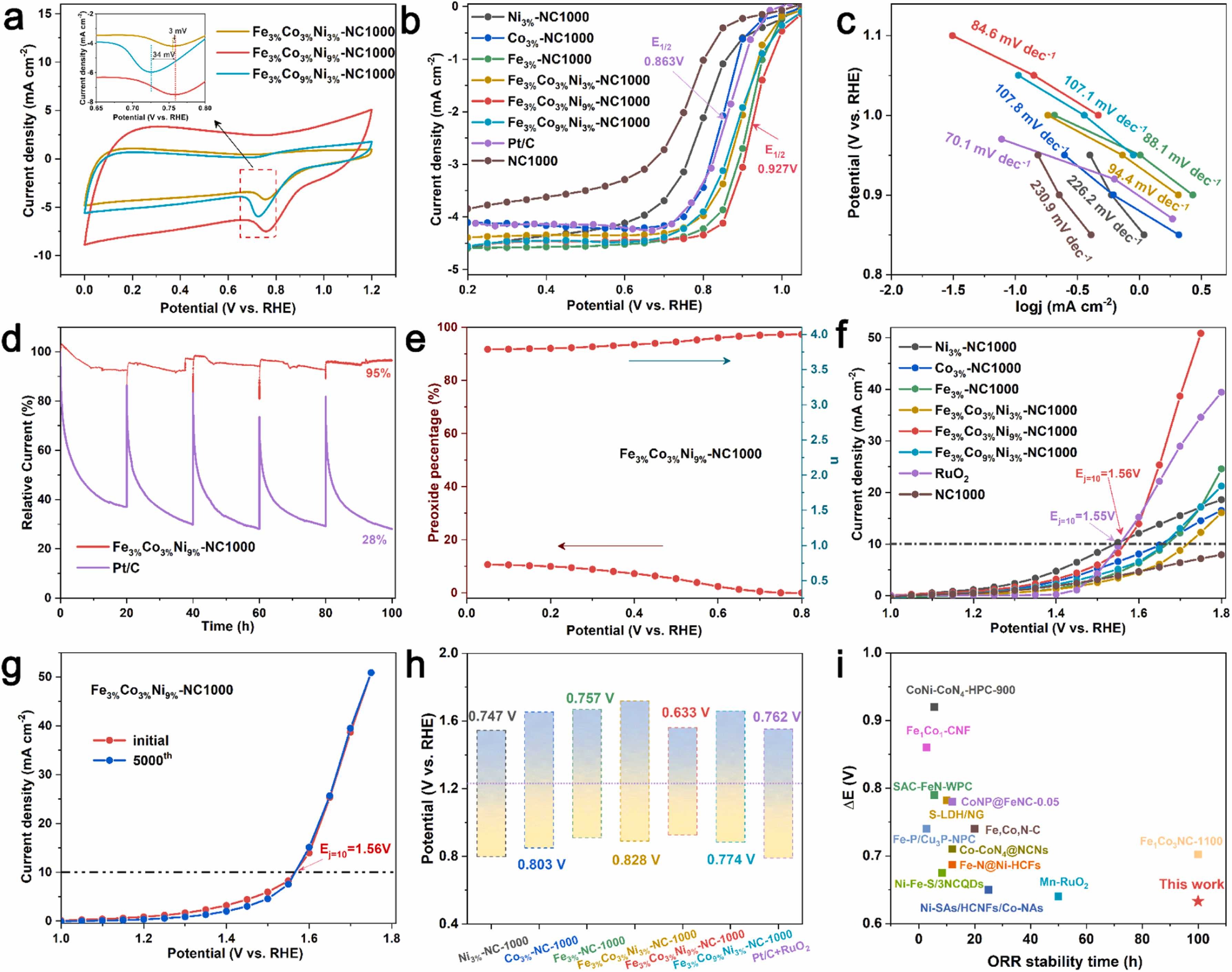
Fig. 4. ORR and OER performance of FexCoyNiz-NC catalysts. a) CV curves at the scan rate of 50 mV s−1. b) ORR curves. c) The corresponding Tafel slope of b). d) Chronoamperometric responses of Fe3%Co3%Ni9%-NC1000 and Pt/C during the ORR at a constant potential of 0.85 V. e) The H2O2 production yields and electron transfer number. f) OER curves. g) Long-term OER stability test of Fe3%Co3%Ni9%-NC1000 and RuO2 at initial and after 5000th CV scanning. h) Comparison of ΔE (ΔE=1.23 V-(η10 +E1/2)) of samples. i) Comparison the ΔE and ORR durability of Fe3%Co3%Ni9%-NC1000 and other reported bifunctional oxygen electrocatalysts.
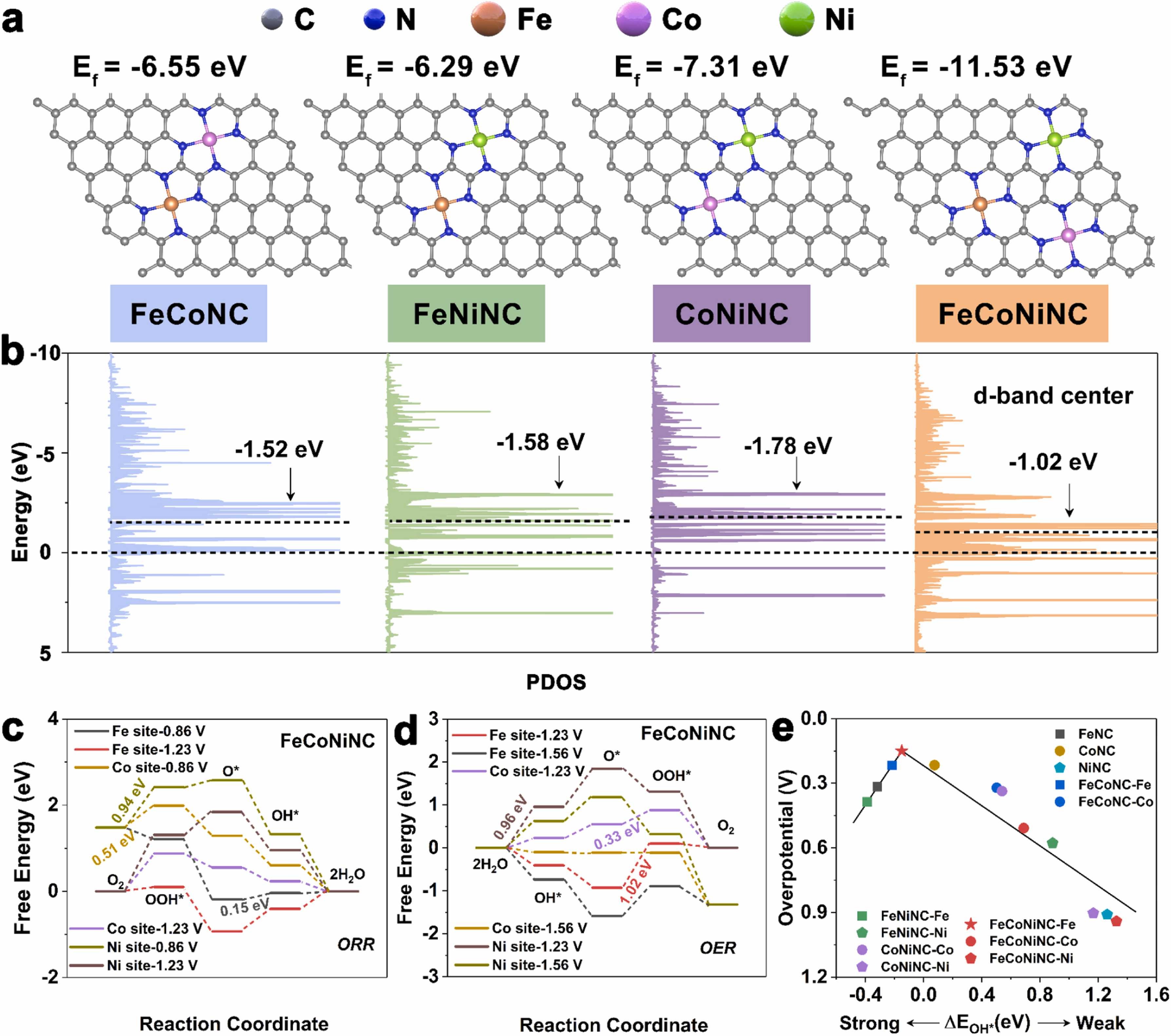
Fig. 5. a) Atomic structures of electrocatalysts. The grey, blue, orange, purple, and green balls represent C, N, Fe, Co, and Ni atoms, respectively. b) Partial density of states (PDOS) and metal d‐band center. c) Free energy for the ORR of FeCoNiNC in different metal-site. d) Free energy for the OER of FeCoNiNC in different metal-site. e) Volcano plot of the ORR overpotential as a function of binding energy for immediate OH*.
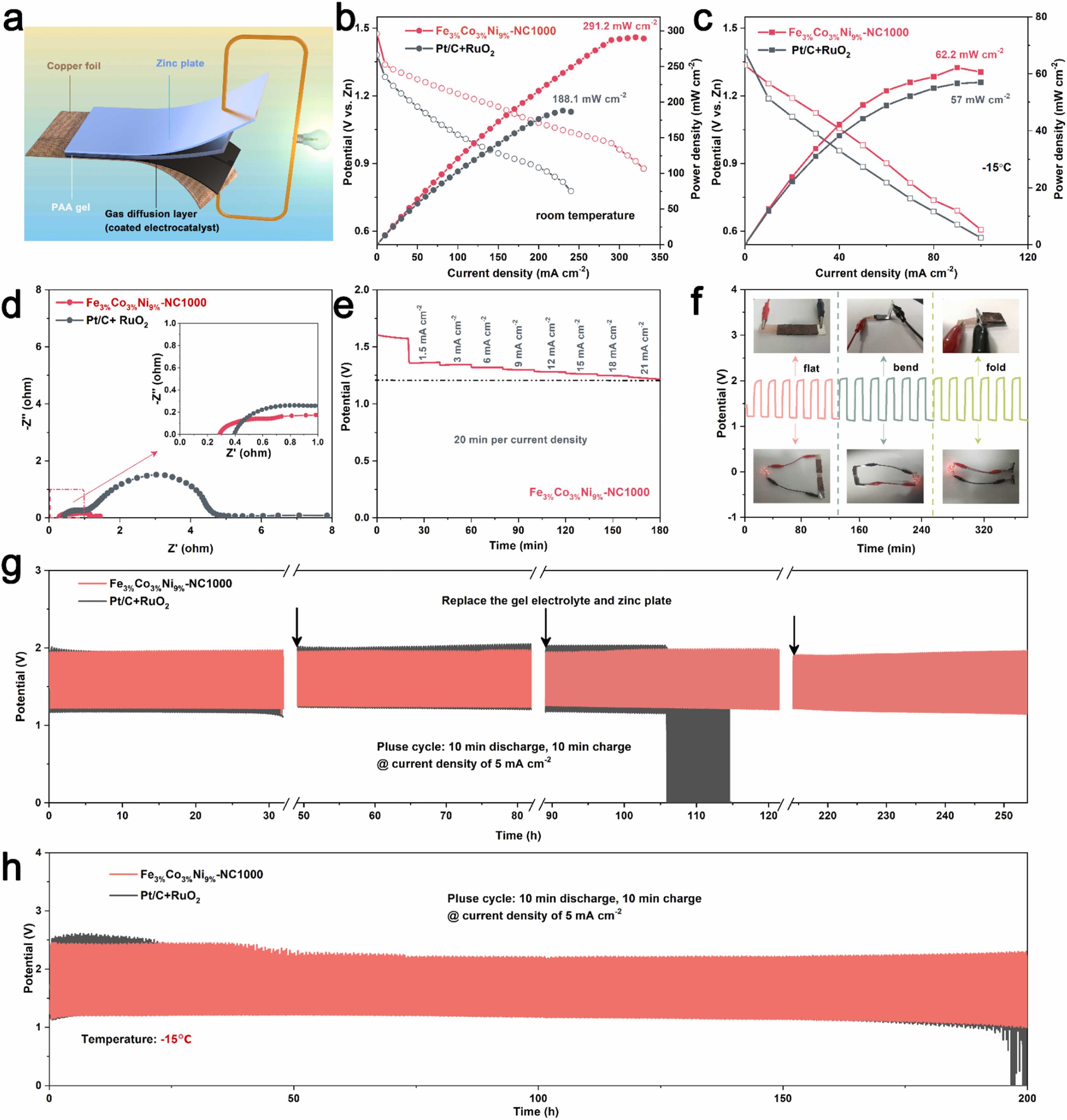
Fig. 6. Performance of FZAB using the Fe3%Co3%Ni9%-NC1000 catalyst. a) Schematic diagram of the Fe3%Co3%Ni9%-NC1000 catalyst-based FZAB. b) Polarization curves and corresponding power density plots at room temperature. c) Polarization curves and corresponding power density plots at −15 ℃. d) Nyquist plots of the impedance of Fe3%Co3%Ni9%-NC1000 and Pt/C+RuO2 catalysts-based FZABs. e) Discharge curves of the FZAB based on Fe3%Co3%Ni9%-NC1000 at different current densities. f) Charge-discharge cycling performance of FZAB based on Fe3%Co3%Ni9%-NC1000 electrocatalyst at a constant charge-discharge current density of 5 mA cm−2 under different bending states. Charge-discharge cycling performance of FZAB based on Fe3%Co3%Ni9%-NC1000 electrocatalyst at different temperatures under constant charge-discharge current density of 5 mA cm−2 g) room temperature, h) −15 °C.
-
2024
03-20
-
2024
03-11
-
2024
03-01
-
2023
11-21
-
2023
10-17
-
2023
10-17

 (创新港)
(创新港)


